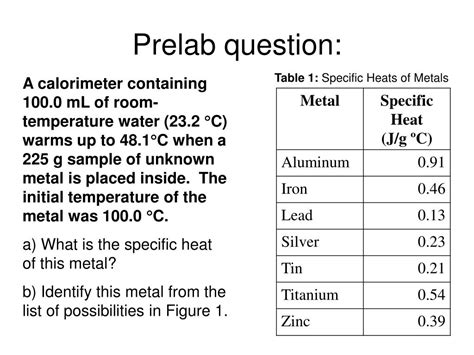
box of specific heat metals for experimt 25 Experiment 25 – Calorimetry (Enthalpies and Specific Heats) Pre-Lab Hints 1. Explain how the temperature of the metal and the water bath become equalized, and how that final equalized . $11.99
0 · experiment 25 calorimetry Flashcards
1 · Specific Heat of a Metal Lab
2 · Solved Experiment 25 Report Sheet Date A. Specific
3 · Solved Experiment 25 Report Sheet Calorimetry Date
4 · Solved "Calorimeting Experiment 25 Report Sheet A.
5 · Experiment 25 – Calorimetry (Enthalpies and Specific Heats)
6 · Experiment 25 Calorimetry Flashcards
7 · Experiment 25
8 · Exp 25 Calorimetry Lab guide
9 · EXP 25
Sigma's weatherproof one-gang boxes provide a junction for conduits and can house a single wired device such as a receptacle or switch. They can also be used to mount lampholders and lighting systems in outdoor applications.
The specific heat of the metal was determined following Part A of the Experimental Procedure in this experiment. Complete the following table for Trial 1 (See Report Sheet.) for determining the specific heat of the metal.Experiment 25 – Calorimetry (Enthalpies and Specific Heats) Pre-Lab Hints 1. Explain how the temperature of the metal and the water bath become equalized, and how that final equalized .
Which of the following is the correct order to perform Part A: Specific Heat of a Metal? step 2 Add __.00 mL water to calorimeter. Record mass of water and calorimeter (without thermometer).Average specific heat of metal /g 'C) Show calculations for Trial I using the correct number of significant figuns. Experiment 25 295 197. Not the question you’re looking for? Post any question and get expert help quickly.Average specific heat of metal (1/80) Data Analysis, B *Show calculations for Trial 1 using the correct number of significant figures. B. Enthalpy (Heat) of Neutralization for an Acid-Base Reaction HCI + NaOH Trial Trial 2 1.
Experiment 25 – Calorimetry (Enthalpies and Specific Heats) Pre-Lab Hints. Explain how the temperature of the metal and the water bath become equalized, and how that final equalized temperature is measured. a. Heat loss causes a .Although the specific heat of a substance changes slightly with temperature, for our purposes, we assume it is constant over the temperature changes of this experiment. The specific heat of a metal that does not react with water is .The purpose of this experiment was to learn how to determine the specific heat of an unknown metal, to determine the enthalpy of a neutralization for a strong acid-strong base reaction, and to determine the enthalpy of a solution for the .In this experiment, you will determine the specific heat of a metal sample. The metal sample will be heated to a high temperature then placed into a calorimeter containing a known quantity of .
-The purpose of the experiment is to calculate the specific heat of a certain metal. This investigation also involves calculating the enthalpy of a neutralization reaction and the .The specific heat of the metal was determined following Part A of the Experimental Procedure in this experiment. Complete the following table for Trial 1 (See Report Sheet.) for determining the specific heat of the metal.
Experiment 25 – Calorimetry (Enthalpies and Specific Heats) Pre-Lab Hints 1. Explain how the temperature of the metal and the water bath become equalized, and how that final equalized temperature is measured. 2. Heat loss causes a lower T f. Examine Figure 25.5 and read the label over the line to see the overall effect on T fWhich of the following is the correct order to perform Part A: Specific Heat of a Metal? step 2 Add __.00 mL water to calorimeter. Record mass of water and calorimeter (without thermometer).Average specific heat of metal /g 'C) Show calculations for Trial I using the correct number of significant figuns. Experiment 25 295 197. Not the question you’re looking for? Post any question and get expert help quickly.
Average specific heat of metal (1/80) Data Analysis, B *Show calculations for Trial 1 using the correct number of significant figures. B. Enthalpy (Heat) of Neutralization for an Acid-Base Reaction HCI + NaOH Trial Trial 2 1.Experiment 25 – Calorimetry (Enthalpies and Specific Heats) Pre-Lab Hints. Explain how the temperature of the metal and the water bath become equalized, and how that final equalized temperature is measured. a. Heat loss causes a lower Tf. Determine how this affects TH2O and TM. Then, determine how those changes affect the result from equation 25.

Although the specific heat of a substance changes slightly with temperature, for our purposes, we assume it is constant over the temperature changes of this experiment. The specific heat of a metal that does not react with water is determined by (1) heat- ing a measured mass of the metal, M. to a known (higher) temperature: (2) placing it into .The purpose of this experiment was to learn how to determine the specific heat of an unknown metal, to determine the enthalpy of a neutralization for a strong acid-strong base reaction, and to determine the enthalpy of a solution for the dissolution of a salt.In this experiment, you will determine the specific heat of a metal sample. The metal sample will be heated to a high temperature then placed into a calorimeter containing a known quantity of water at a lower temperature.
experiment 25 calorimetry Flashcards
-The purpose of the experiment is to calculate the specific heat of a certain metal. This investigation also involves calculating the enthalpy of a neutralization reaction and the disintegration of salt.The specific heat of the metal was determined following Part A of the Experimental Procedure in this experiment. Complete the following table for Trial 1 (See Report Sheet.) for determining the specific heat of the metal.
steel city cabinet saw price
Experiment 25 – Calorimetry (Enthalpies and Specific Heats) Pre-Lab Hints 1. Explain how the temperature of the metal and the water bath become equalized, and how that final equalized temperature is measured. 2. Heat loss causes a lower T f. Examine Figure 25.5 and read the label over the line to see the overall effect on T fWhich of the following is the correct order to perform Part A: Specific Heat of a Metal? step 2 Add __.00 mL water to calorimeter. Record mass of water and calorimeter (without thermometer).
Average specific heat of metal /g 'C) Show calculations for Trial I using the correct number of significant figuns. Experiment 25 295 197. Not the question you’re looking for? Post any question and get expert help quickly.Average specific heat of metal (1/80) Data Analysis, B *Show calculations for Trial 1 using the correct number of significant figures. B. Enthalpy (Heat) of Neutralization for an Acid-Base Reaction HCI + NaOH Trial Trial 2 1.Experiment 25 – Calorimetry (Enthalpies and Specific Heats) Pre-Lab Hints. Explain how the temperature of the metal and the water bath become equalized, and how that final equalized temperature is measured. a. Heat loss causes a lower Tf. Determine how this affects TH2O and TM. Then, determine how those changes affect the result from equation 25.Although the specific heat of a substance changes slightly with temperature, for our purposes, we assume it is constant over the temperature changes of this experiment. The specific heat of a metal that does not react with water is determined by (1) heat- ing a measured mass of the metal, M. to a known (higher) temperature: (2) placing it into .
The purpose of this experiment was to learn how to determine the specific heat of an unknown metal, to determine the enthalpy of a neutralization for a strong acid-strong base reaction, and to determine the enthalpy of a solution for the dissolution of a salt.In this experiment, you will determine the specific heat of a metal sample. The metal sample will be heated to a high temperature then placed into a calorimeter containing a known quantity of water at a lower temperature.
Specific Heat of a Metal Lab
Solved Experiment 25 Report Sheet Date A. Specific
15 Amp Outdoor Electrical Outlet Box with On/Off Switch and Indicator Light, 125 Volt NEMA 5-15 Duplex Receptacle, Grounding, IP66 Waterproof Wall Power Socket, Weather and Tamper Resistant
box of specific heat metals for experimt 25|Experiment 25 – Calorimetry (Enthalpies and Specific Heats)